
MS 4525 - C420 Unit 2 Homework
Solutions can be seen at mendelset.com/ms/4525
Description: Second homework set for Chem 420
Total Problems: 13
-
Problem # 1287
Rank each set of compounds in order of decreasing boiling point (1 = highest boiling point):
a) ethane, n-octane, n-pentane
b) n-butane, 1-butanol, 1-chlorobutane.
c) n-octane, 2-methylheptane, 2,5-dimethylhexane
(Note that the n- prefix before an alkane just means that it's one chain, without any branching.)
-
Problem # 315
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!

-
Problem # 310
For each molecule, determine the formal charge of the indicated atom.

-
Problem # 311
For each molecule below, draw in all implied lone pairs and/or protons (hydrogens) based on the formal charge shown.

-
Problem # 313
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!

-
Problem # 569
Draw all resonance forms for each species.
For the anion and cation species, used curved arrows. For the radical species, use hooks.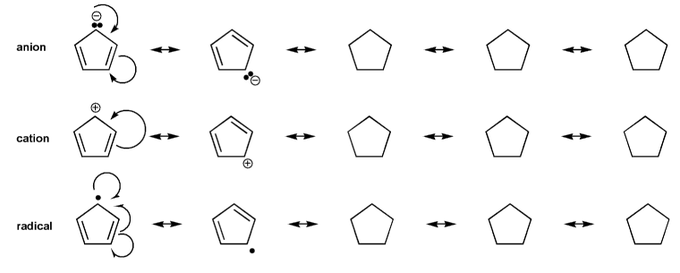
-
Problem # 580
Draw all resonance forms for each species.
For the anion and cation species, used curved arrows. For the radical species, use hooks.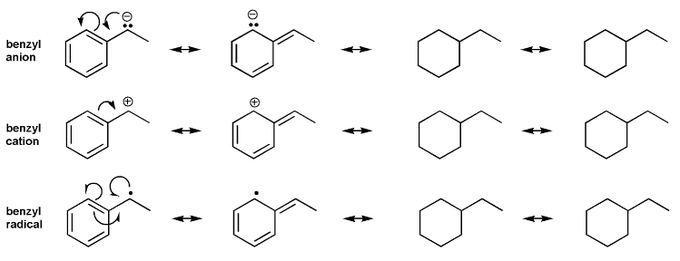
-
Problem # 697
Rank the following compounds in order of decreasing boiling point.
Also, make a guess about their relative solubilities in water. Explain your reasoning.
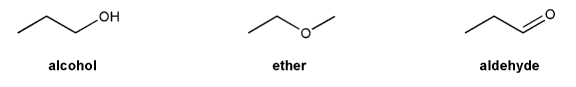
-
Problem # 535
Rank the following anions in order of decreasing stability (1 = most stable)
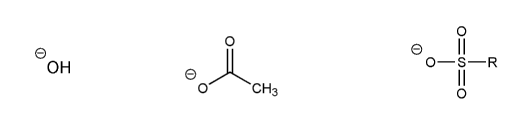
-
Problem # 307
Rank the group of molecules below in in order of decreasing basicity. (1 = most basic)
Explain your reasoning.

-
Problem # 540
Rank the following anions in order of decreasing stability (1 = most stable)

-
Problem # 286
Draw the conjugate base forms of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.

-
Problem # 288
Draw the conjugate base form of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.
