MS 915 - NMR Practice
Solutions can be seen at mendelset.com/ms/915
Description: Several NMR problems. Two are conceptual and the rest are structure determination.
Total Problems: 7
-
Problem # 730
N,N-dimethylformamide (DMF) is shown below. Based on its structure, you might expect to see only one -CH3 signal in the 1H NMR spectrum. But instead DMF shows two different -CH3 signals. Explain.

-
Problem # 661
The proton NMR of cyclohexane gives only one peak when the NMR is run at room temperature.
But when the temperature is lowered to -100 ºC the proton NMR spectrum shows two peaks. Explain.
-
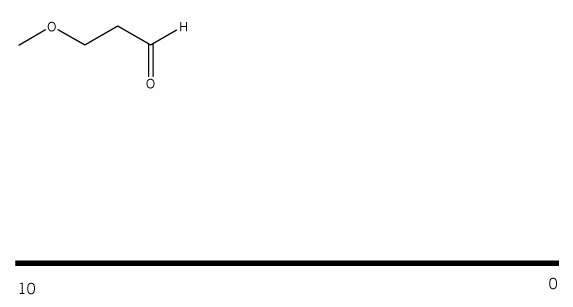
Problem # 660
Using your knowledge of 1H NMR, predict the NMR spectrum for the compound below. (draw out the spectrum you would expect to see). Be sure to include:
- peak integrations
- peak multiplicities
- chemical shifts (approximate)
-
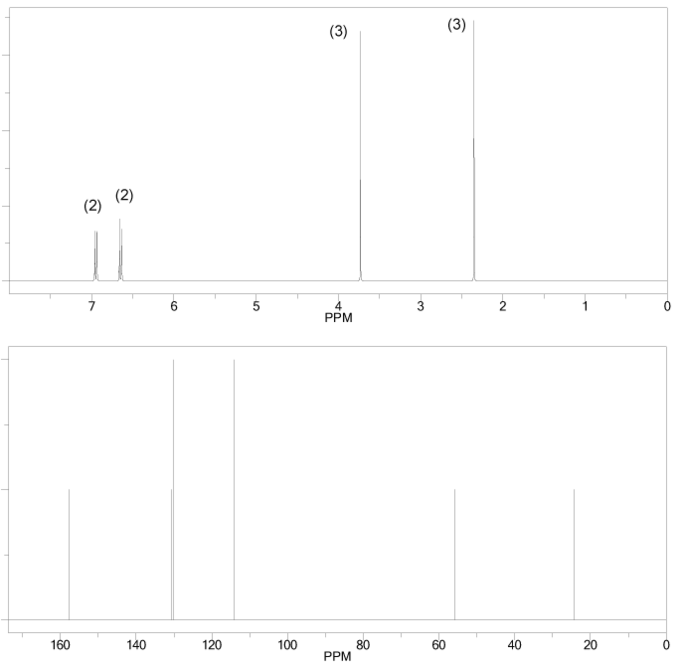
Problem # 662
The 1H and 13C NMR spectra of a compound with chemical formula C10H14O are shown below. The compound's IR spectrum shows a broad peak at 3,300 cm-1. Determine the structure of this compound.
-
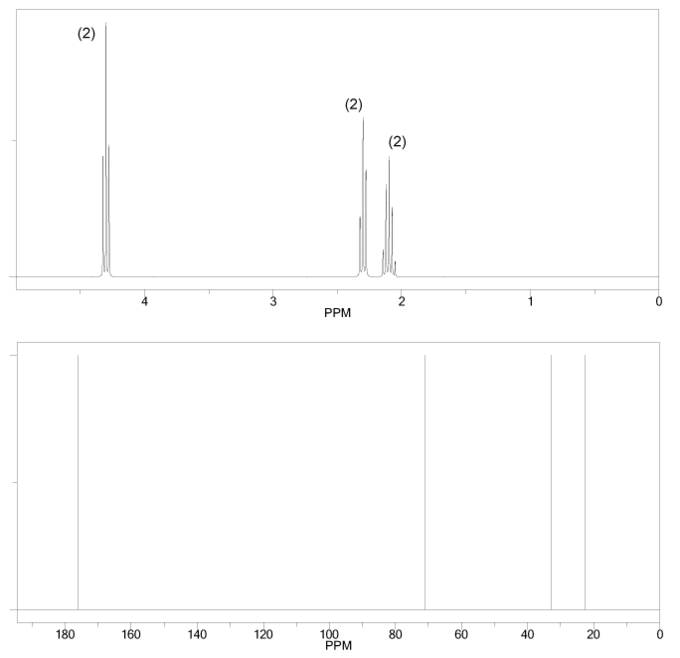
Problem # 663
The 1H and 13C NMR spectra of a compound with chemical formula C4H6O2 are shown below. The compound's IR spectrum shows a sharp peak at 1,700 cm-1. Determine the structure of this compound.
-
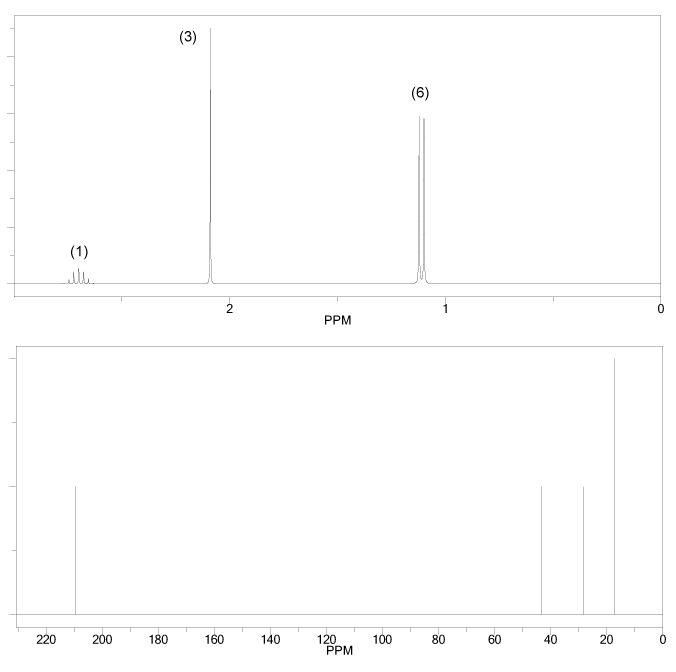
Problem # 665
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 86. Determine the structure of this compound.
-
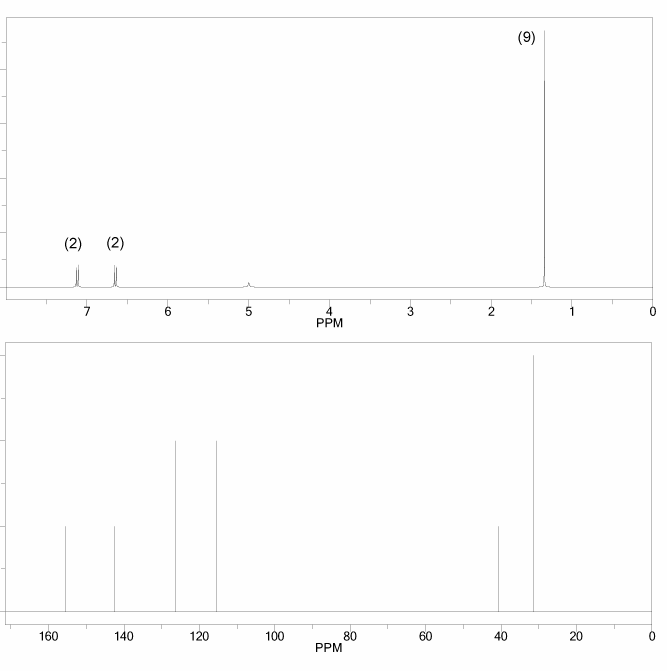
Problem # 666
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 122. Determine the structure of this compound.