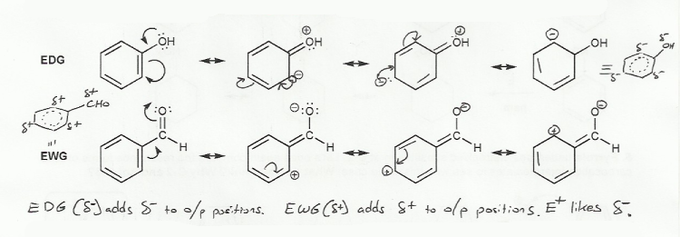
The resonance forms for EDG add electron density to the ring (and add a negative charge), while the resonance forms for EWG remove electron density from the ring (and leave a positive charge). This is where the terms electron donating group and electron withdrawing group come from.
EAS reactions require benzene to attack something positive (an electrophile), so the more electron density, the better. This is why EDG tend to speed up EAS reactions, while EWG slow down EAS reactions.
Notice that for EDG, the ortho and para positions are partially negative. In EAS reactions benzene attacks a positively charged electrophile, so its not too surprising that the electrophile will want to add at o/p if a EDG is present.
But for EWG, the o/p positions pick up a partial positive charge. So for EAS reactions with a EWG, it's not surprising that the electrophile will avoid the o/p positions, and add meta instead.
This is one explanation for the general trend:
-
EDG are ortho/para directors and activating.
-
EWG are meta directors and deactivating.