There's only one major concept you need to know for melting points:
Impure samples have lower melting points than pure samples.
And that's just about all you need to know...
Contents:
How to Take a Melting Point
The first step in taking a melting point is to pack your solid sample into a capillary tube. What’s a capillary tube you ask? It’s a narrow, hollow glass rod that’s closed at one end. You only need to place a little bit of sample in the tube- this is best done by “stabbing” your sample a few times. Then tap the tube gently to make the solid fall to the bottom.
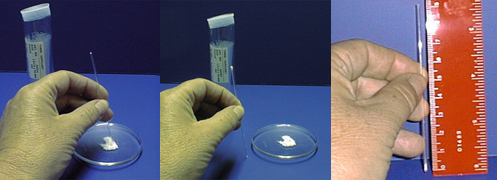
Once you have a few millimeters worth of sample in the capillary tube place it in your melting point apparatus (Mel-Temp for short). Once you turn it on the Mel-Temp will start to heat up. The idea is to record the temperatures at which the solid begins and finishes melting.
Key Melting Point Concepts
Melting Point Range
We say “melting point”, but solids really melt over a temperature range.
For a pure substance the range is narrow- usually only a degree or two (Celsius). If you have a very broad melting point range that means your sample isn’t very pure.
How Fast to Allow the Mel-Temp Temperature to Rise?
To get an accurate melting point measurement you should allow the Mel-Temp to rise in temperature slowly- about 1-2 ºC each minute.
Of course- this is painfully slow. So you shouldn’t slow down to this rate of heating until you’re 10-20º away from the melting point.
What to do if you don’t know what the melting point is before hand? (For example, you have an unknown sample, or you’re not sure how pure your sample is).
Do a quick melting point first- let the temperature rise quickly, just so you have an idea of what the melting point is. Then do a slow and careful melting point afterward.
Decomposition and Sublimation
You can’t take the melting point of every solid. Some substances skip the liquid phase and go straight from solid to gas. This is called sublimation.
Other solids decompose at high temperatures. For example, you try to take the melting point of a white crystalline solid and it turns black- this is decomposition.
Questions You Will Probably Be Asked
Q: The melting point of pure 3-nitrobenzoic acid is 139-141 ºC. How would this compare with an impure sample of 3-nitrobenzoic acid?
A: An impure sample will have a lower melting point and a broader melting point range. So a possible value is 110-120 ºC.