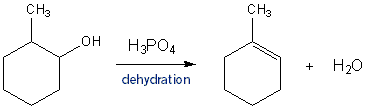
This week you will be preparing an alkene by dehydrating an alcohol. This reaction proceeds via the same mechanism as in Carey CH 5.9, which I will review in detail.
Contents:
The Dehydration Mechanism
Dehydration takes place in two main parts:
- Converting the –OH to a better leaving group and then forming the carbocation.
- Removing a beta-hydrogen to convert the unstable carbocation to a stable alkene.
There is also the possibility of a carbocation rearrangement. Let’s go over each of these parts.
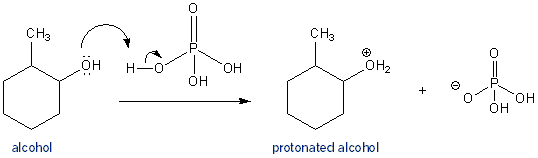
Protonation of the Alcohol Group
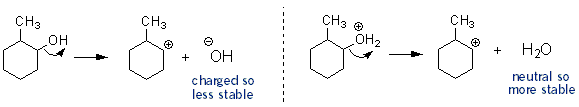
An alcohols (-OH) is not a very good leaving group, because after it dissociates from a molecule it becomes a negatively charged hydroxide ion. Far better would be to first protonate the alcohol. This way it leaves the molecule as water- which has no charge and is very stable. Phosphoric acid gets this job done. When the protonated alcohol dissociates from the molecule it leaves behind a carbocation.
Carbocation Rearrangement
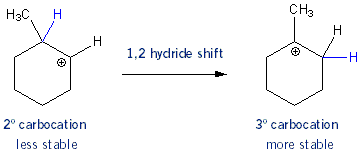
After the protonated alcohol leaves the molecule you are left with a secondary carbocation. But this secondary carbocation could become the more stable tertiary carbocation if the hydrogen- bond and all- moved over from the adjacent tertiary carbon. This is in fact what happens- the carbocation undergoes a 1,2 hydride shift. It turns out that this isn’t too important- as you’ll see in a moment both 2º and 3º carbocations give the same major alkene product.
Beta-Hydride Elimination to Form the Alkene
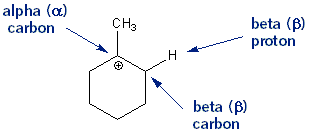
Carbocations don’t hang around very long because they’re unstable. One way to get rid of them is for a base to rip off a proton (hydrogen) from a carbon one-way from the carbocation- a beta proton (β proton). This forms an alkene. This process is called beta elimination. When there is a choice of different beta protons to remove the most highly substituted alkene is the major product.
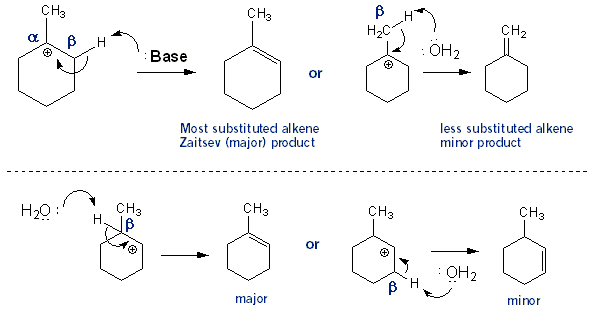
Key Dehydration Concepts
Dehydration is Reversible
Dehydration is a reversible reaction- under the same conditions (water in the presence of acid), alkenes can be converted right back into alcohols (see Carey CH 6.10). So how can you prevent the reverse reaction? Two ways:
- Use a weaker acid, such as H3PO4 instead of H2SO4. This will make the reverse reaction less likely.
- Remove water from the reaction as it is generated. This can be hooking up your reaction to a vacuum line, so the water is sucked out of your reaction as it forms and it isn’t available to add to the alkene. (You don’t do this in this lab).

Getting All Sorts of Side Products
Because the reaction is reversible, it’s possible that the following situation can happen: The alcohol dehydrates to your major alkene product, which then forms a different carbocation, which then dehydrates to a different alkene, which then forms a different carbocation, and so on. While this can happen, it’s not too likely, and these other alkenes will only account for a very small proportion of your products. But this is the explanation for why you get some side products.

E1 vs. E2
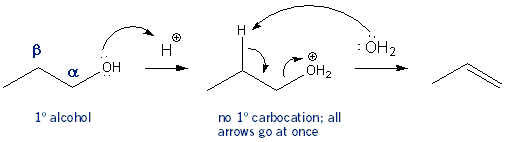
Dehydration usually goes via an E1 mechanism. That is, the reaction takes place over two steps, the first being the formation of a carbocation intermediate. But if you had a primary alcohol the reaction wouldn’t go through a carbocation intermediate. This is because a 1º carbocation is too unstable to form. The reaction would all happen all at once in one step- it would go through an E2 mechanism.
Plain English Procedure
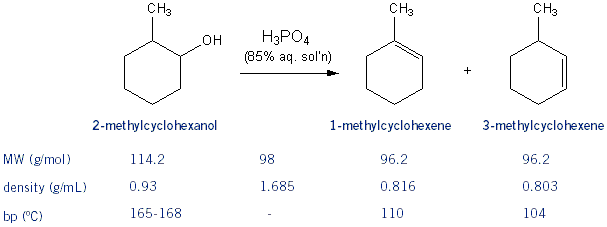
You’re going to add 2-methylcyclohexanol (~6 mL) and phosphoric acid (~5 mL) in a round bottom flask with a boiling stone. You will then do a fraction distillation setup to run the reaction/collect your product.
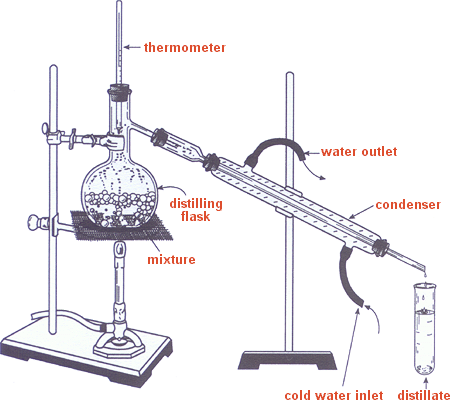
Keep the temperature below 96 ºC. Yes, this is below the boiling points of your products, however, because boiling works through vapor pressure, you don’t have to be at the boiling point to collect your product. You will collect about 4-6 mL of product, which will include some water. Because cyclohexene is immiscible with water it will form layers; your product will be the upper layer (because it’s less dense than water). Use a pipette to collect the upper layer and transfer it to another piece of glassware (your text suggests a centrifuge tube). Add some sodium sulfate (NaSO4) and let it sit for ~10 minutes. The sodium sulfate will suck up any water that might be with your product. Finally, you’re going to analyze your product using GC. You can estimate the approximate ratios of major to minor products by comparing the peak areas on the GC print out.
