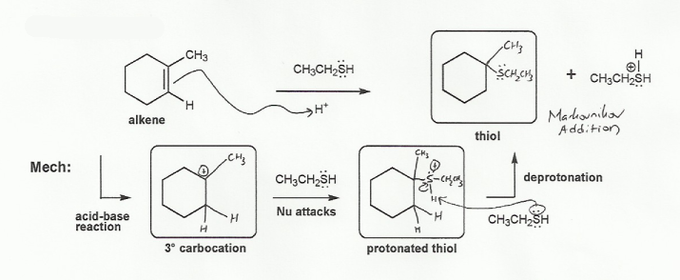
Note that this is the reverse of problem 519. Instead of going from thiol (alcohol) to carbocation to alkene, we're going from alkene to carbocation to thiol. In each step ask yourself "what arrows can I draw?" and choose the step that doesn't go backwards.
The first step is the alkene picks up a proton to form the more stable carbocation. (See problem 334 if you don't understand why only the 3º carbocation is formed). To get rid of the carbocation, we can either do a beta-elimination (E1) to form an alkene, or an addition reaction (SN1) to form a protonated thiol. Since forming the alkene would be going backwards, the only choice is addition.
Then you're left with a protonated thiol. How do we get rid of the positive charge on the sulfur? There are two legal moves- either the RSCH2CH3 acts as a leaving group, or the sulfur deprotonates. Since the RSCH2CH3 leaving would form a carbocation and take us backwards, the only option is for the sulfur to deprotonate.
Because the sulfur added to the more substituted carbon, this was a Markovnikov addition.