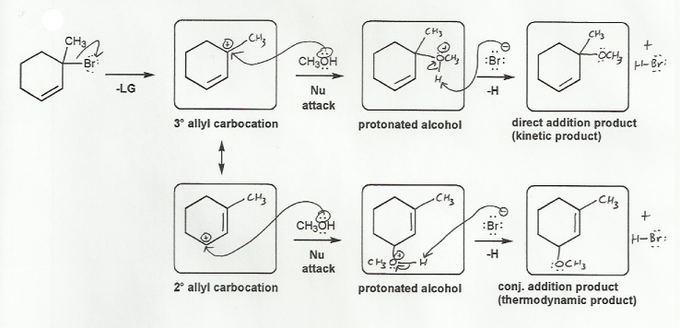
This is an SN1 reaction, but with a twist.
The top reaction is a regular SN1 reaction; the leaving group (Br-) leaves and the nucleophile (CH3OH) attacks the carbocation.
The twist is that the carbocation is allylic, and has resonance, so there is another carbon that the nucleophile can attack.
So instead of just one SN1 product, two products are formed. The product that doesn't involve resonance is called the direct addition or 1,2 product. The product that involves allylic resonance is called the conjugate addition or 1,4 product.