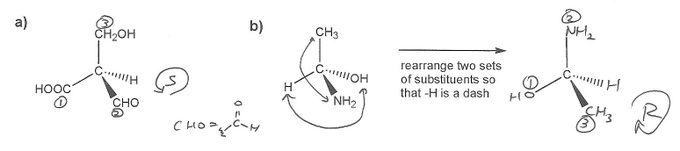
It's easiest to assign R or S configuration when the lowest priority substituent is "in the back" or "behind" the molecule, that is, a dash. Most of the time the lowest priority substituent will be a hydrogen atom.
So a) is straightforward. Because hydrogen is already a dash, we can ignore it and see in which direction the other three substituents decrease in priority (according to Cahn–Ingold–Prelog priority rules). Since they decrease in a counter-clockwise way, a) has S absolute configuration.
b) is a little harder to assign. If hydrogen were a wedge we would be able to just take the opposite of whatever answer we get. But in this case, H is neither a wedge nor a dash. So we use a trick: if we swtich any two pairs of substituents, the absolute configuration of the molecule remains the same. So we switch two pairs so that the hydrogen becomes a dash. On the resulting molecule, the priorities 1-3 decrease in a clockwise manner, so this molecule has an R absolute configuration.