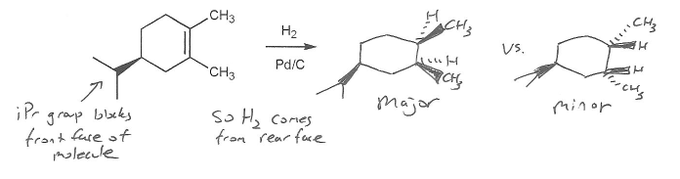
This is a hydrogenation reaction. The product is an alkane. But this product has two stereocenters.
Normally, the product would be a racemic mixuture of enantiomers (equal amounts of each stereoisomer). But in this case, the starting material is optically active (chiral but not racemic), so the products won't be equal and opposite as usual.
Since there is a substitutent blocking the top face of the molecule (isopropyl is a wedge), the hydrogens will preferentially add to the opposite face of the molecule (dashes).