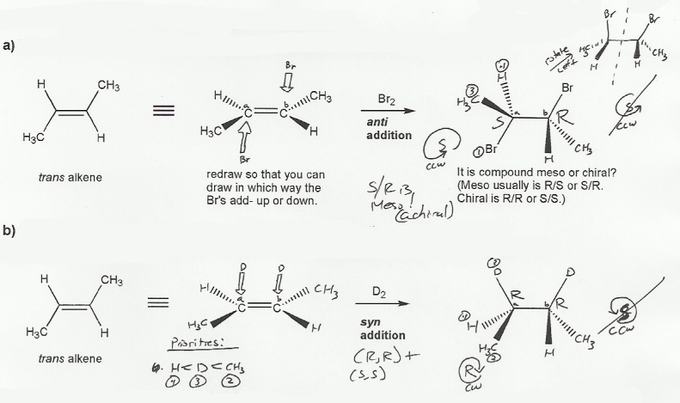
The reaciton of an alkene with Br2 is an anti-addition, and hydrogenation (H2 or D2) is a syn addition. From this we can figure our the relative stereochemistry of each product.
Each starting material is achiral, and therefore not optically active, so the products cannot be optically active.
There are three ways for products not to be optically active:
-
products can be achiral
-
products can be a racemic mixture
-
products can be meso
In both a) and b) each product has a sterocenter, so the products can't be achiral.
In a), one stereocenter is S and the other stereocenter is R, and both with the same substituents, so this is a mirror image relationship, and the products are meso.
In b), the products have no internal mirror planes, so the products are chiral, and must be a racemic mixture.