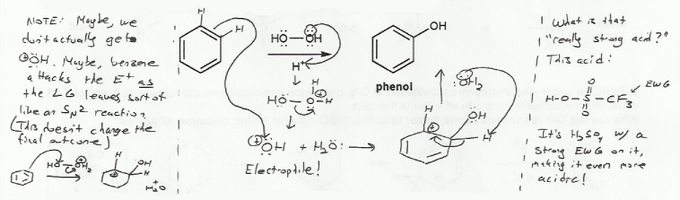
The electrophile we need for this EAS reaction is HO+. We can generate it by protonating one of the oxygens in peroxide, which causes it to act as a leaving group.
On the left side of this image I make a note about the structure of the electrophile. Some textbooks use electrophiles with formal positive charges, such as HO+. Other textbooks don't use formal positive charges, and instead use partial positive charges with a leaving group, such as HO-OH2+. Using HO+ as the electrophile is analogous to an SN1 reaction, while using HO-OH2+. is analogous to an SN2 reaction. When you draw mechanisms, you should use whichever convention your textbook uses. Both lead to the same product.