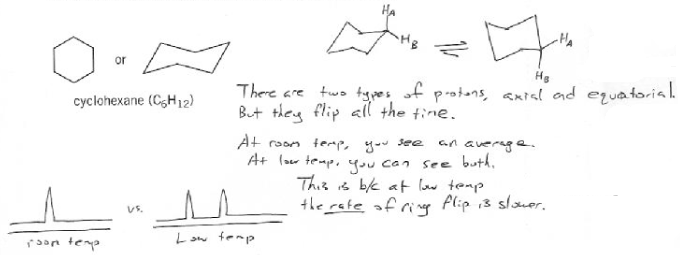
Every proton on cyclohexane appears identical, but remember than cyclohexane is usually in a chair form, so there are really two types of protons: axial and equatorial.
In theory each of these two protons should give its own NMR peak. But at room temperature the molecule is undergoing chair flip so rapidly the two peaks converge into one.
But at low temperature the rate of chair flip slows down enough that two distinct peaks emerge.