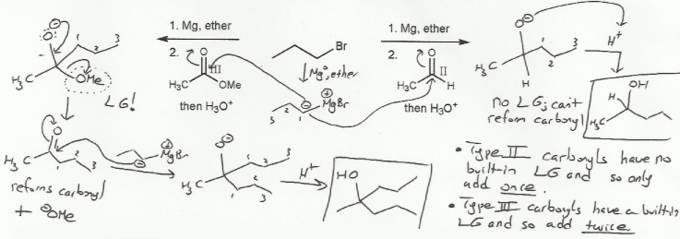
Oxidation state III carbonyls (esters, acid chlorides) contain a built-in leaving group (such as -OR or -Cl) and so undergo nucleophilic acyl substitution reactions. The product is another carbonyl.
Oxidation state II carbonyls (aldehydes and ketones) do not contain a built-in leaving group and so undergo nucleophilc acyl addition reactions (instead of substitution). The product is an alcohol.
Because Grignards react with all carbonyls- esters and aldehydes/ketones- esters and acid chlorides will react twice with Grignards: once in a Nuc Acyl Sub mechanism to form a ketone, which will then react with another equivalent of Grignard in a Nuc Acyl Add mechanism to form an alcohol.