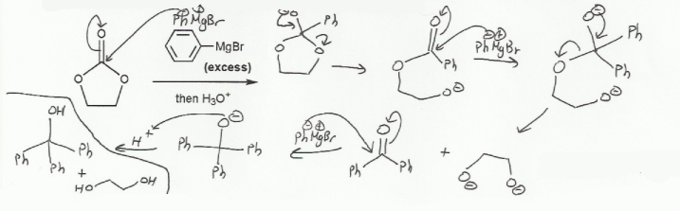
This carbonyl has two leaving groups attached to it- each of those oxygens can take part in a nucleophilic acyl substitution reaction and form a new carbonyl product.
First the Grignard attacks the oxidation state IV carbonyl carbon (4 oxygen bonds, so oxidation state 4). The carbonyl itself will act as a leaving group and form a tetrahedral intermediate. But tetrahedral intermediates don't last if there are any leaving groups attached to the carbon, so the -O will "come back down again", kick off an oxygen leaving group, and reform the carbonyl.
Then a second equivalent of Grignard will attack that carbonyl (an ester), and we will do another nucleophilic acyl substitution reaction to form yet another carbonyl.
Finally, the third carbonyl doesn't have any leaving groups built in (it's a ketone), so when the third equivalent of Grignard attacks it, it will do a nucleophilic acyl addition reaction, and the product will be an alcohol.
Notice that as the reaction progresses the oxidation state of the carbonyl carbon (number of oxygen bonds attached to it) goes down form 4 to 3 to 2 and then to 1.