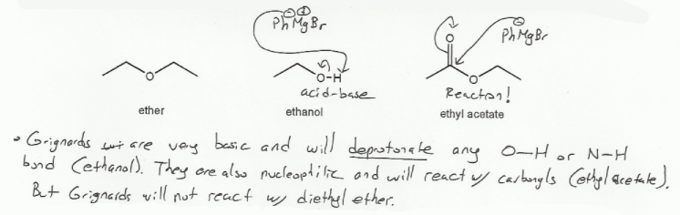
Grignards behave as though they are carbanions (negatively charged carbons), and so are very basic. There are so basic that they will deprotonate any O-H or N-H bond. So protic solvents such as water or ethanol aren't suitable for Grignard reactions; the Grignard reagent will react with the alcohol in an acid-base reaction.
In fact, water is used after a Grignard reaction to quench the Grignard reagent.
Grignards are also nucleophilic, and so react with carbonyls (which are electrophiles).
Ethyl acetate contins a carbonyl and would get attacked by a Grignard reagent, and so also isn't a suitable solvent for a Grignard reaction.
Diethyl ether doesn't have any acidic protons and isn't electrophilic and so won't react with a Grignard reagent, so it makes a good solvent.