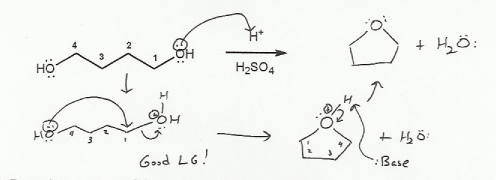
This is just an SN2 reaction.
The first step is to turn one of the -OH groups into a better leaving group by having it pick up a proton. (I choose the hydroxyl on the right side).
Then the other -OH group can attack carbon 1, and the protonated hydroxyl group leaves as water.
Finally, the proton on the -OH group that acted as a nucleophile will "fall off" (deprotonate).