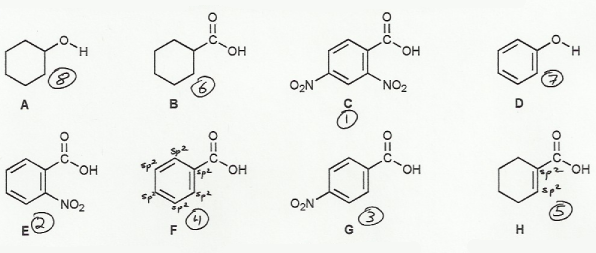
Carboxylic acids are much more acidic than alcohols (because of resonance in their conjugate bases), so A and D are the least acidic of the group. The conjugate base of D has resonance, so D is more acidic than A:
(everything) > D > A
Electronegative atoms increase acid strength (by stabilizing the conjugate base), and sp2 carbons are more electronegative than sp3 carbons (due to higher s-character). So B (all sp3 carbons) will be the least acidic of the carboxylic acids, and H will be more acidic than B (two sp2 carbons):
(everything) > H > B > D > A
Of the remaining 4 benzoic acids, C has the most EWG (two nitros) and so is the most acidic. F has no EWG and so is the least acidic. Both E and G have only one EWG and so will be similar in acidity, with E slightly more acidic than G because the EWG affect is strongest in the ortho position (closer to the action). So the overal order is:
(strongest acid) C > E > G > F > H > B > D > A (weakest acid)