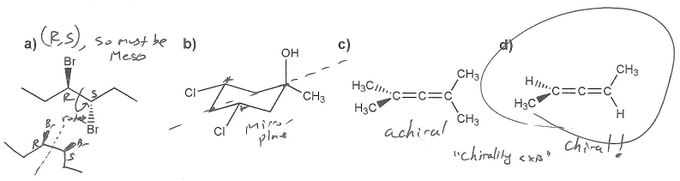
There are several types of chirality. In undergraduate organic chemistry, most chiral molecules exhibit point chirality- they have at least one sterocenter and don't have a plane of symmetry. Molecules a) and b) both have stereocenters, but they also both have planes of symmetry, so neither is chiral (they are both meso compounds).
Molecule can also be chiral about an axis. The classic example of this is allenes- molecules with two consecutive double bonds. Compound c) has a plane of symmetry so it can't be chiral. It might be hard to see, but compound d) is in fact chiral- it's mirror images are non-super impossible. It has a "chirality axis." Just like a screw can be right-handed or left-handed, so can molecule d).