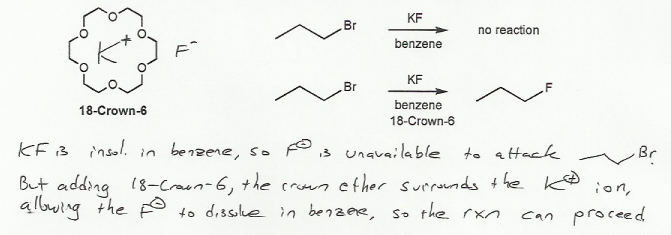
KF is insoluble in propyl bromide or benzene, so the two compounds never "touch" each other, and no reaction takes place. (F- and propyl bromide are in different phases and so don't come into contact).
But when the crown ether is added, it solvates the potassium ion, allowing the K+ and F- ions to dissolve in the solvent, so the fluoride ion and propyl bromide are able to "talk" to each other, and the rection can take place.