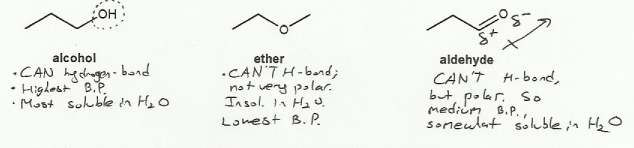
Water is very polar, so the more polar a molecule is, the more soluble it will be in water.
Alcohols can hydrogen bond, and so will be the most polar, have the highest boiling point, and be the most soluble in water.
Aldehydes (carbonyls) can't hydrogen bond but the C=O double bond is very polar, so aldehydes are somewhat soluble in water.
The ether is the least polar of the three functional groups, and so will have the lowest boiling point, and be the least soluble in water.
So the overal trend for polarity, water solubility, and boiling point is:
alcohol > aldehyde > ether