To be a stereocenter you need to:
-
be a carbon
-
be sp3 hybridized (all single bonds)
-
have four different substituents
Are there other types of stereocenters? Of course! ( sp3 sulfur atoms, for example). But in undergraduate organic chemistry, 99% of all sterocenters you come across will be sp3 hybridized carbon atoms.
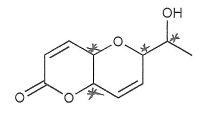
So in the molecule below, there are four stereocenters.
Note that the sp2 (alkene) carbons can't be sterocenters.