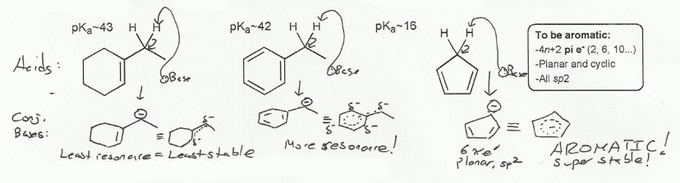
The benzylic proton (middle compound) is more acidic than the allylic proton (left compound) because its conjugate base is more stable. This is because it has more resonance forms.
Cyclopentadiene (right compound) is the strongest of the three because it has the most stable conjugate base. Why is it the most stable? Because it's aromatic! To be aromatic, a compound must:
-
be cyclic and planar
-
be sp2 hybridized
-
Have a Huckel number of pi electrons- 2, 6, 10, 14, etc.
The cyclopentadienyl anion meets all of these criteria, and so is aromatic, and very stable.