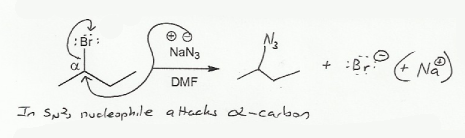
Arrows in organic chemistry always go from regions of high electron density to regions of low electron density. Most of the time this means arrows start from negative charges and go towards positive charges.
Because bromine is electronegative, the carbon directly bonded to it (also known as the alpha carbon) has a partial positive charge, and can be attacked by a nucleophile such as azide (N3-).
Because this is an SN2 reaction, no carbocation is formed; as the nucleophile attacks the alpha carbon, the leaving group (Br-) leaves.