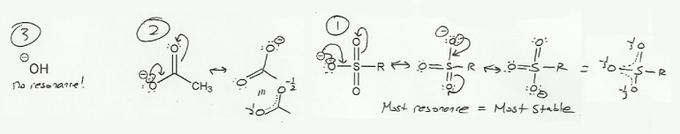
Charged molecules are generally less table than neutral ones, and each of the molecules below has a negatively charged oxygen.
But not all negative charges are equal; some oxygens are "closer to neutral" than others. How? Because resonance stabilizes charges by sharing electron density over multiple atoms (this is called electron delocalization).
Hydroxide (HO-) doesn't have resonance, so the oxygen has 100% of the negative charge to itself. On the other hand, the sulfonate ester (SO3R-) has three resonance forms, so each oxygen only has ~33% of a negative charge. So the sulfonate ester is the most stable anion.
In general, the more resonance forms a molecule has, the more stable it is.