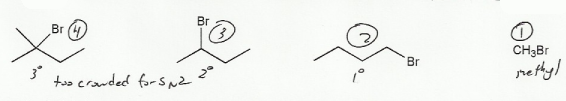The only differences among these compounds is the substitution of the alpha carbon (methly, 1º, 2º, or 3º).
This is an SN2 reaction. We know this because NaI is an SN2 reagent- charges give it away; when we see charges (Na+ is positive and I- is negative) it's probably an SN2 reaction. So we want reactants that are less substituted: methyl is more reactive than 1º, which is more reactive than 2º, etc. So methyl bromide reacts the fastest with NaI.