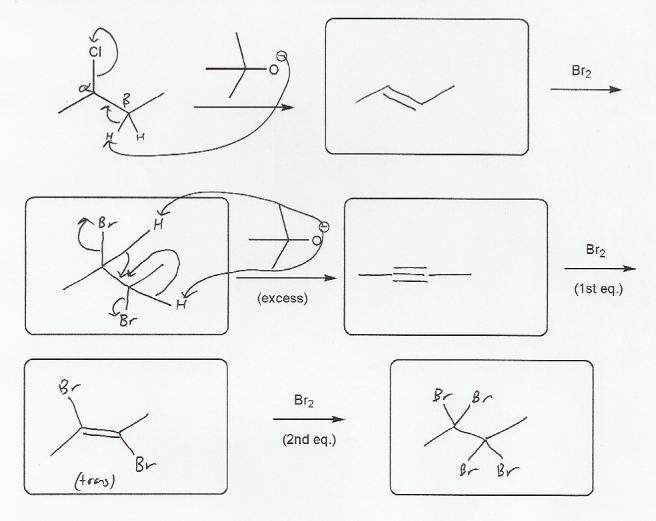
-OtBu is a big, bulky base, and so will do an E2 reaction with the alkyl halide. The elimination product can be either a cis or trans alkene, but trans is usually the major product (cis alkenes have steric strain).
The alkene then reacts with Br2 to form 2,3-dibromobutane, which can undergo two consecutive E2 reactions with -OtBu to form an alkene and then the alkyne (2-butyne).
Finally, the alkyne reacts with the first equivalent of Br2 to form the trans 2,3-dibromo-2-butene, which reacts with a second equivalent of bromine to form the tetrabromo alkane.