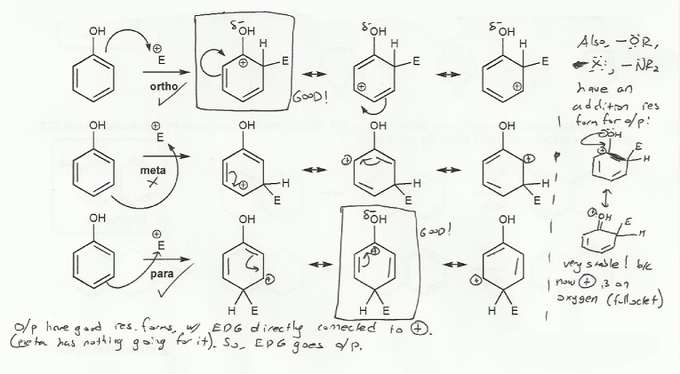
Short answer: -OR is an ortho/para director because the cyclohexadienyl intermediates that result from ortho and para addtitions are more stable than those that result from meta addition.
Long answer: The cyclohexadienyl intermediates from ortho and para addition include a resonance form where the oxygen is adjacent to the carbocation, while the cyclohexadienyl intermediates from a meta addition do not.
When an oxygen (or anything with a lone pair) is adjacent to a carbocation, it can share its lone pair and stabilize the positive charge. This is only possible when the electrophile adds to the ortho or para positions, so those positions are favored with -OR as a substituent. Hence, -OR is an ortho/para director.
Note that this logic holds for any substituent with a lone pair, so -OH, -OR, -NH2, -NR2, -F, -Cl, -Br, and -I are all ortho/para directors.