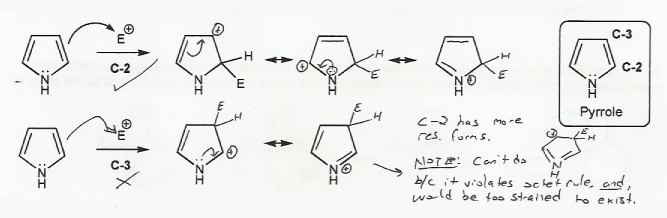
Electrophilic substitution at C-2 leads to a carbocation intermediate with three resonance forms, while substitution at C-3 leads to a carbocation intermediate with only two resonance forms.
The C-2 intermediate has more resonance forms than the C-3 intermediate, and so is more stable. Therefore, EAS occurs at C-2.