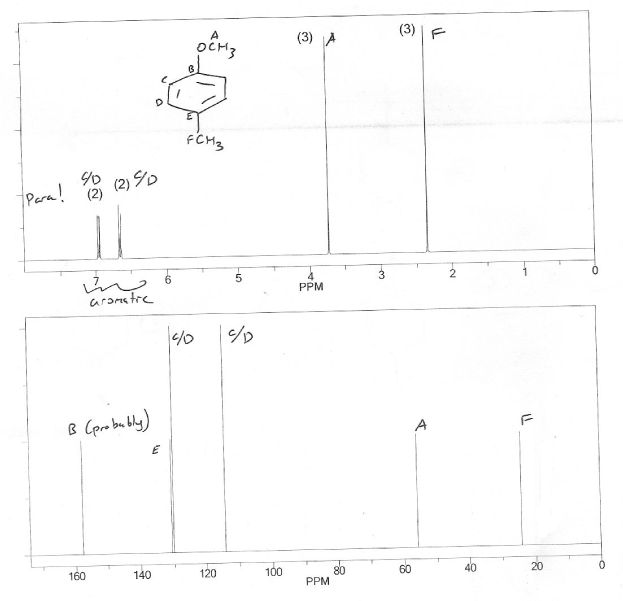
This is similar to problem 665- a very difficult NMR problem because we're not given the unknown's molecular formula, only its molecular mass (122 from the molecular ion peak on the mass spec).
So the first thing we have to do is guess the molecular formula based on this mass. Here's my thought process:
-
There are signals in the aromatic region on the 1H NMR (~7 ppm). So we probably have at least 6 carbon's with at least 4 IHD's (a benzene ring has 4 IHD).
-
There are two other signals on the 1H NMR, so there are probably at least 2 more carbons, for 8 carbons in total.
-
8 carbons with 4 IHD would give a formula of C8H10 (C8H18 from CnH2n+2 minus 8 H's for the 4 IHDs). which only gives a mass of 106. But we need a mass of 122- we're 16 mass units short. That's exactly one oxygen!
-
Based on this, C8H10O is a good guess for the formula.
Now we can follow the steps laid out in problem 662:
1. Are there any hints? & 2. How many IHD are there?
Mass spec NMR problems require us to have done these two steps by now.
3. Draw some C8H10O structures with 4 IHD and eliminate, learn, repeat.
Some things we know form the NMR spectra:
-
Because there are only four protons in the aromatic region (total integration of 4), the benzene ring is disubstituted. The two peaks in the aromatic region (~7 ppm) are doublets with integrations of 2, so this ring is probably para substituted.
-
The benzene ring "uses" 6 carbons which leaves 2 carbons for the other peaks. They're probably both methyls because they both have integrations of 3. They're also both singlets, so there's not adjacent to any other carbons that have a hydrogen. The peak that's further downfield (1H ~3.8 ppm) is probably near the oxygen.
Draw a few structures based on these clues, and eventually you will come to the correct structure.