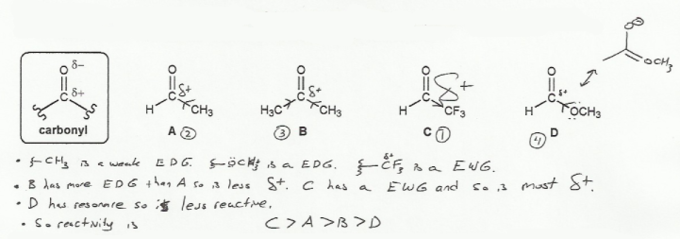
The carbonyl carbon is electrophilic because it has a partial positive charge.
Are there any groups that make a carbon more positive? Yes, electron withdrawing groups (EWG). They pull away electron density, which increases electrophility. So C is the most reactive.
Conversely, electron donating groups (EDG) add electron density, and so make the carbonyl carbon less positive, and less electrophilic. Alkyl groups (carbon chains) are mildly EDG so ketone B will be less reactive than C.
Hydrogen is neither a EDG or EWG, so the aldehyde A will be in between the B and C. Also, the hydrogen is very small, so the carbonyl carbon is easily to get to (less steric bulk to block an attack).
Ester D is the least reactive because it has a resonance (the lone pair on the oxygen gets involved).
So overall, the order form most reactive to least reactive is C > A > B > D.