Let's solve this NMR structure elucidation problem using steps similar to those used in problem 662.
1. Are there any hints?
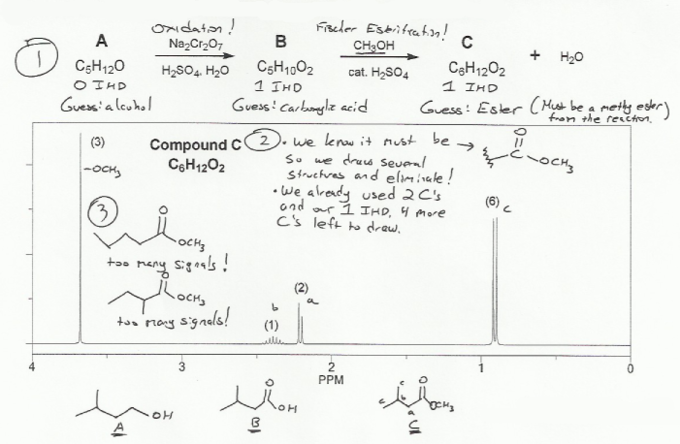
Compound A has one oxygen and after treatment with aqueous chromium becomes compound B, which has two oxygens. This means A is probably an alcohol, B is probably a carboxylic acid.
Compound B is then treated with methanol under acidic conditions to form compound C. These are conditions for a Fischer esterification, so C is probably the methyl ester.
2. How many IHD are there?
Compound A: C5H12O = C5H12 should be C5H12 (CnH2n+2) so 0 IHD.
Compound B: C5H10O2 = C5H10 should be C5H12. Missing 2H, so 1 IHD.
Compound C: C6H12O2 = C6H12 should be C6H14. Missing 2H, so 1 IHD.
These IHD counts fit our assumptions from part 1).
3. Draw some structures and eliminate, learn, repeat.
Some clues from the NMR:
-
The isopropyl splitting pattern is present: d(6) (signal c at ~0.9 ppm) and multiplet(1) (signal b at ~2.4 ppm).
-
The s(3) at ~3.7 ppm is probably the methyl group from the methyl ester.
-
We know from before we have one IHD, and it's probably an ester.
So start drawing structures and eliminate those that don't fit the data!