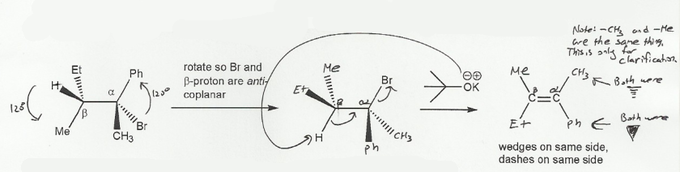
To predict the stereochemistry of the alkene product from an E2 reaction, we have to rotate the leaving group (Br) and the beta-proton into anti-coplanar geometry. It's easiest to put the H and Br in the plane of the page.
After we do this, we see that both the ethyl and phenyl groups are wedges, and the two methyls are dashes. So after the E2 reaction, the resulting alkene will have its two methyl groups cis to each other.