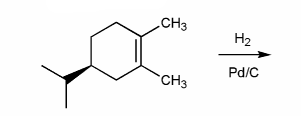
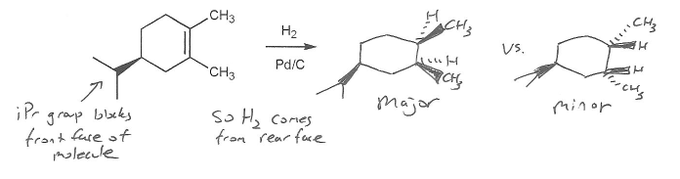
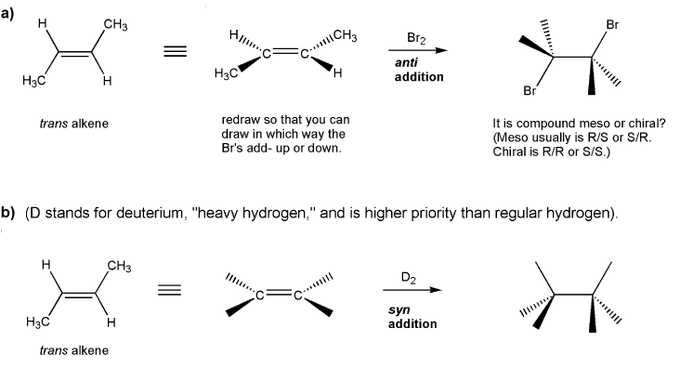
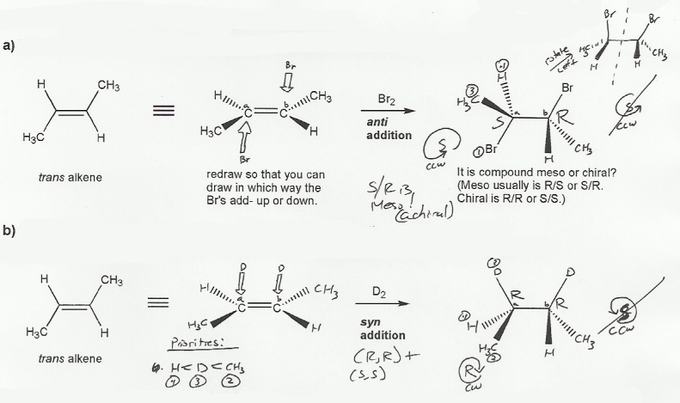
Textbook: Carey and Giuliano 8th Ed. (2010)
Chapter 6: Addition Reactions of Alkenes
Practice Problems and Mendel Sets
Individual Problems
Mendel Sets
Textbook and Chapters: Carey and Giuliano 8th Ed. (2010), Chapters 4, 5, 6
Keywords: alkene addition, carbocation
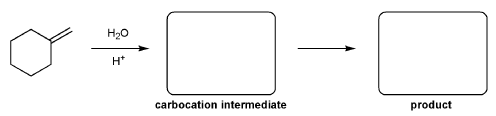
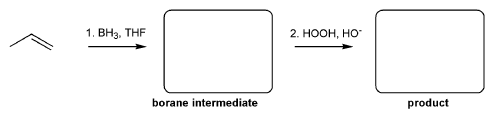
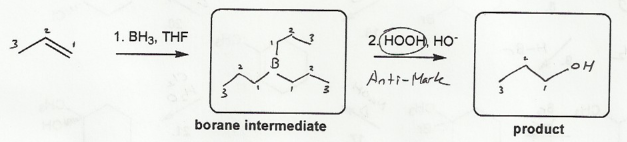
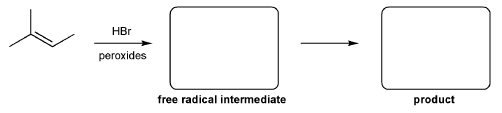
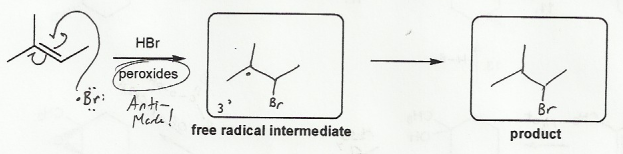
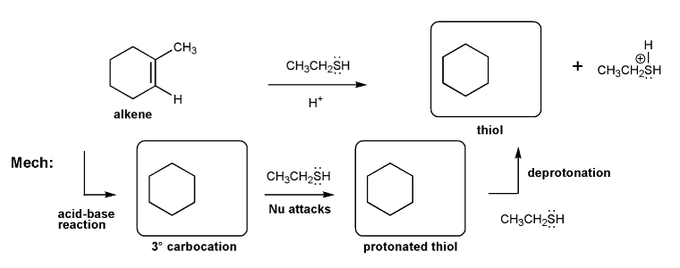
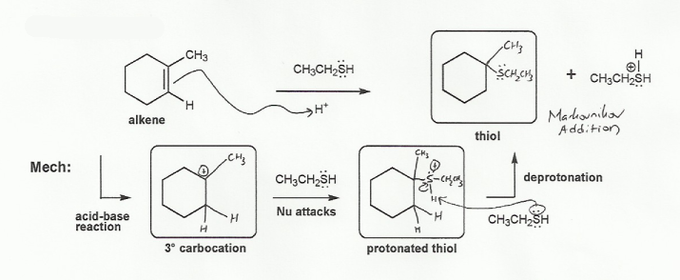
Description: Identify the intermediates (carbocation, radical, borane intermediate, etc.) and products for important reactions dealing with alkenes. Good review for an orgo1 midterm.
Total Problems: 7
Textbook and Chapters: Carey and Giuliano 8th Ed. (2010), Chapters 4, 5, 6
Keywords: carbocation, carbocation formation, carbocation rearrangement
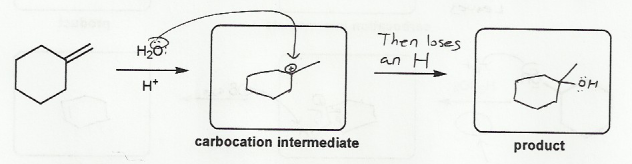
Description: This mendel set guides you through everything you have to know about carbocations:
- Ways carbocations form
- Carbocation rearrangements
- How carbocations react (elimination or nucleophilic attack)
Also includes some practice problems: addition to an alkene, dehydration (E1), and substitution (SN1).
Total Problems: 8
Textbook and Chapter: Carey and Giuliano 8th Ed. (2010), Chapter 6
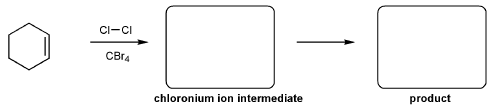
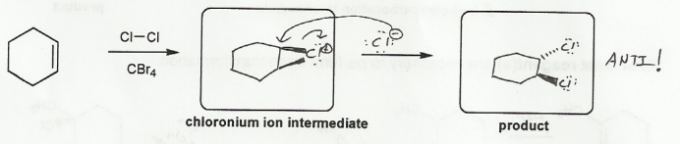
Keywords: alkene addition, carbocation, dehydration, E1 mechanism
Description: This mendel set is a complement to Carbocation Drills. It is meant to prepare students on how to approach longer and more complicated mechanisms. Reaction mechanisms covered:
- Acid-catalyzed elimination (dehydration, E1)
- Acid-catalyzed addition to an alkene
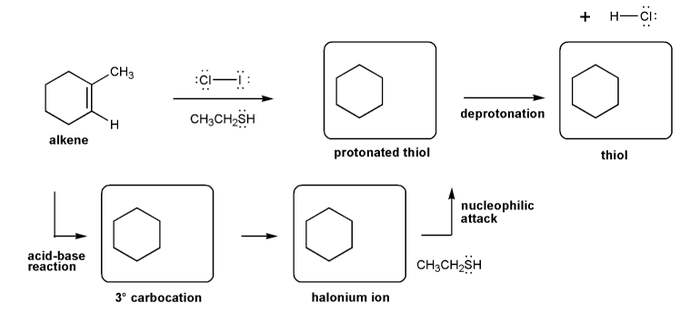
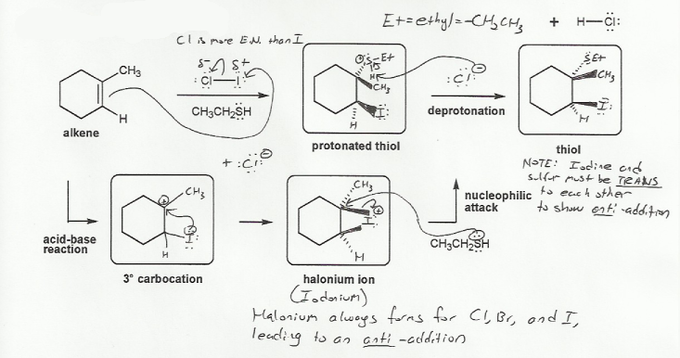
- Halohydrin formation (halonium ions, which result in anti-additions.)
Total Problems: 4