Textbook: Carey and Giuliano 8th Ed. (2010)
Chapter 15: Alcohols, Diols, Thiols
Practice Problems and Mendel Sets
Individual Problems
Mendel Sets
Textbook and Chapter: Carey and Giuliano 8th Ed. (2010), Chapter 15
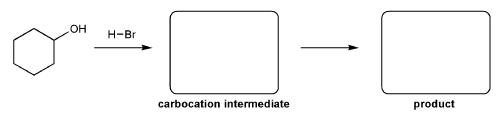
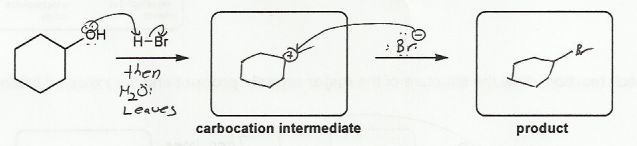
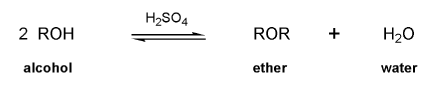
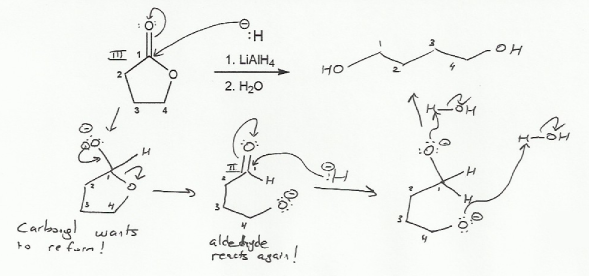
Keywords: alcohols, hydride reduction, oxidation
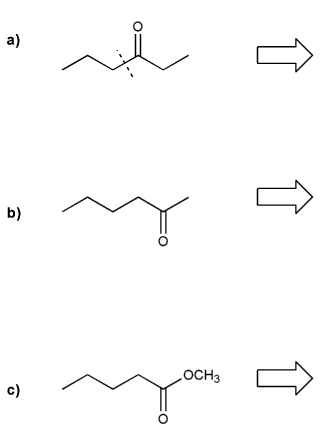
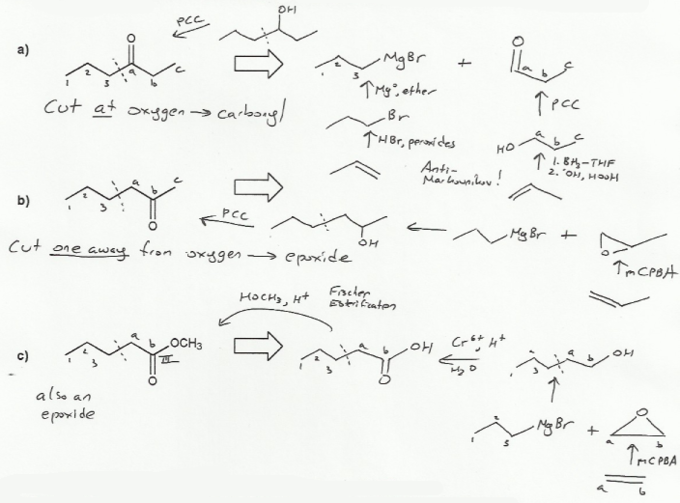
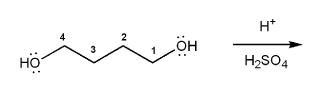
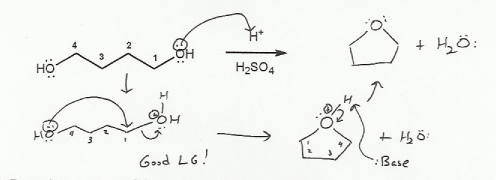
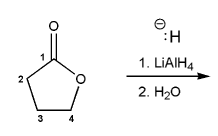
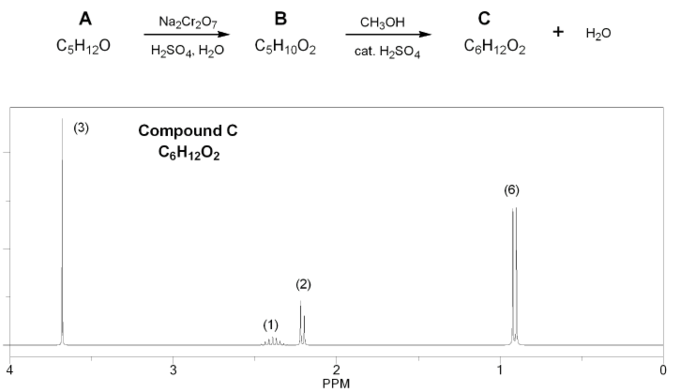
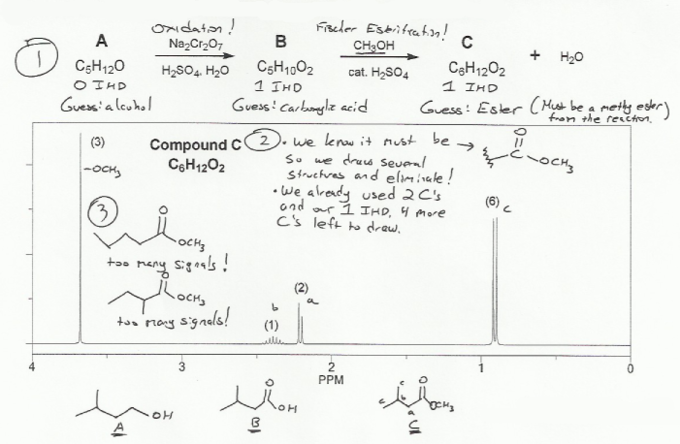
Description: Goes over how alcohols can be oxidized to form aldehydes/ketones and carboxylic acids, which can be transformed further using Grignard reagents, hydride reagents such as NaBH4, and by performing Fischer esterification. Includes several synthesis problems involving carbonyls and epoxides. Also includes an NMR based problem.
Total Problems: 5