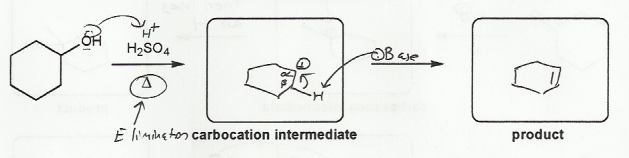
The delta (Δ) in the reaciton arrows means that heat is being added to this reaction, which tends to favor elimination over substitution. Also, the reaction is using a non-nucleophilic acid (H2SO4), which tends to favor elimination reactions (H3PO4 is another common reagent for E1 reactions, while HCl or HBr tend to go SN1).
Because this reaction is taking place in acid, a carbocation is likely to form, so this is an E1 reaction. Since water is lost over the course of the reaction, this is a dehydration, which is a type of elimination reaction.